
Poster Exhibition Area
Vivacitas Oncology, Inc.

Authors
Sotirios Bisdas, M.D., Ph.D.
Jai S. Grewal, M.D.
Volker W. Stieber, M.D.
Robert Cavaliere, M.D.
Craig E. Devoe, M.D.
Randy L. Jensen, M.D., Ph.D.
Elise Brownell, Ph.D.
Tina Runk, M.B.A.
Diana Dupont-Roettger, Ph.D.
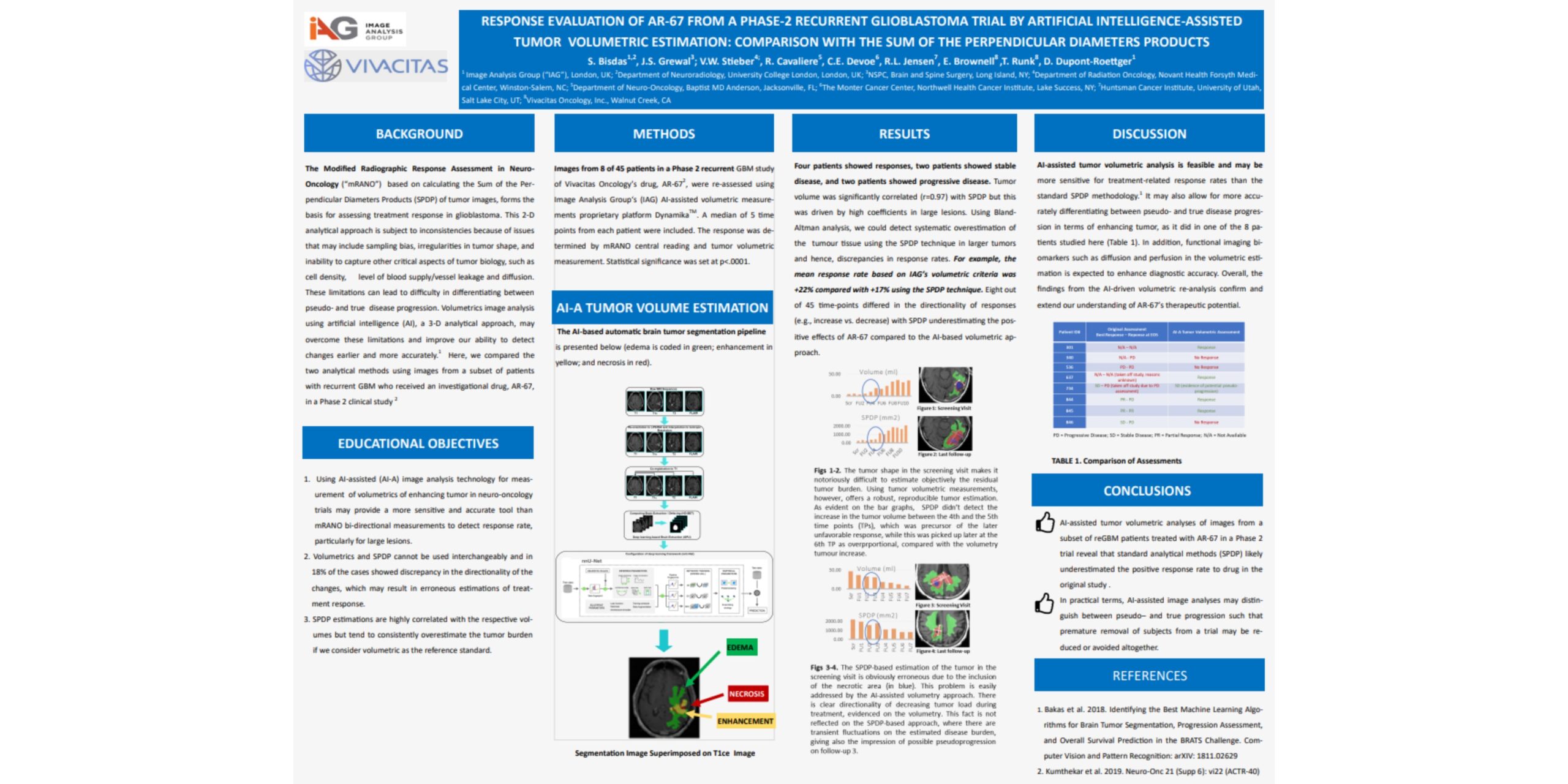
Response evaluation of Vivacitas Oncology’s drug, AR-67 from a Phase-2 recurrent Glioblastoma (“reGBM”) trial by IAG’s Artificial Intelligence (“AI”)-assisted tumor volumetric estimation: a comparison with the sum of the perpendicular diameters product (“SPDP”)
Modified Radiographic Response Assessment in Neuro-Oncology (“mRANO”) criteria based on SPDP have traditionally formed the basis for assessing treatment response in Glioblastoma but show variability due to sampling bias of the complex tumor morphology and difficulty in determining between progression of disease and pseudo-progression. Volumetrics image analysis using Artificial Intelligence (“AI”) may overcome limitations of bi-dimensional measurements and improve our ability to detect changes earlier, and more robustly and accurately.
Hemispherian AS

Authors
Terezia Prikrylova,
Adam Robertson,
Zeno Albisser
Hemispherian AS, Gaustadalleen 21, Oslo, 0349, Norway
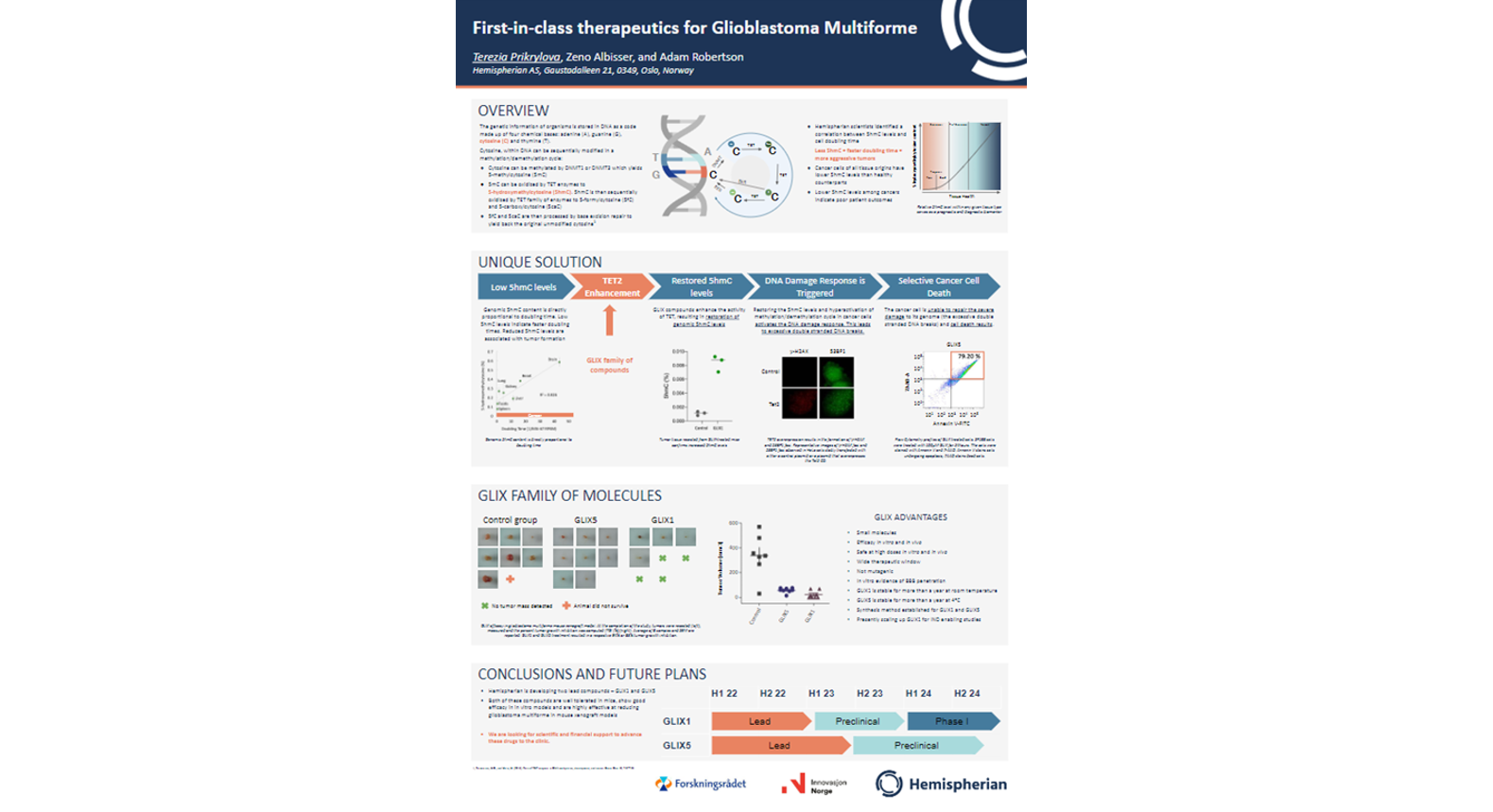
First-in-class therapeutics to treat Glioblastoma Multiforme
GLIX compounds make use of the DNA damage response to selectively induce cancer cell death
Hemispherian is advancing first-in-class therapeutics for cancers that are historically difficult to treat. Glioblastoma multiforme (GBM) is the primary indication for our lead compound GLIX1, which is currently in late-stage preclinical development. In combination with GLIX1 a biomarker is under development to pair diagnostic and optimal treatment. It is Hemispherian’s mission to provide superior treatment options for brain cancer patients.
The GLIX mechanism of action targets the base excision repair pathway, by enhancing the activity of the TET2 enzyme in cancer cells. This results in an increase in the levels of genomic 5-hydroxymethylcytosine. The increase in 5-hydroxymethylation hyperactivates the DNA damage response causing selective cancer cell death. GLIX molecules have limited effects on healthy cells as normal levels of 5-hydroxymethylcytosine are maintained.
The preclinical data collected to this date shows promising results with regards to efficacy and tolerability, including in cell lines known to be resistant to the currently available frontline treatment.
Advocate Aurora Health

Authors
Samuel D. Zwernik,
Beau H. Adams,
Dan Raymond,
Catherine M Warner,
Richard A. Rovin,
Parvez Akhtar Advocate Aurora Research Institute, Milwaukee, WI
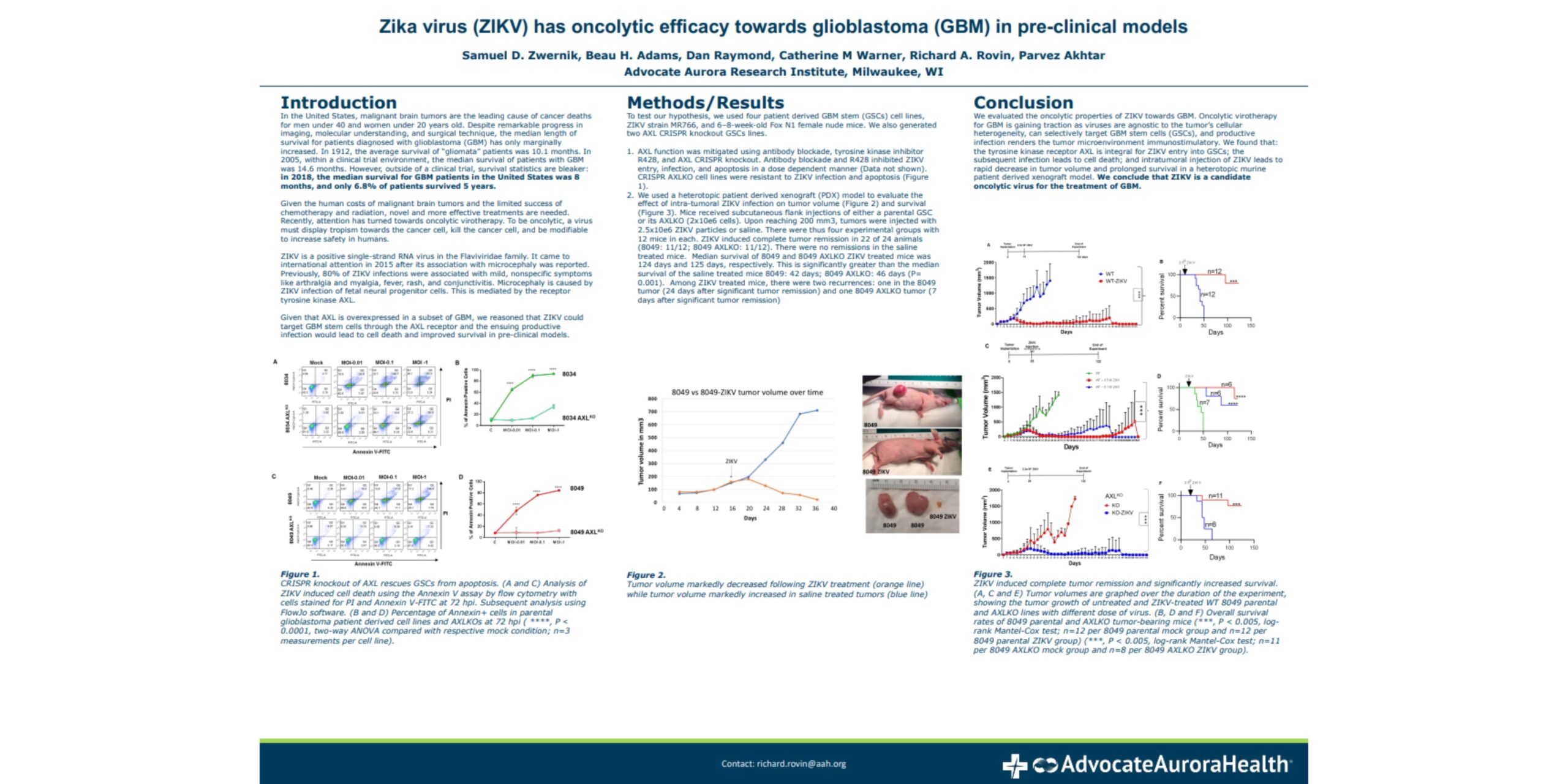
Zika virus (ZIKV) has oncolytic efficacy towards glioblastoma (GBM) in pre-clinical models
We evaluated the oncolytic properties of ZIKV towards GBM. Oncolytic virotherapy for GBM is gaining traction as viruses are agnostic to cellular heterogeneity, can selectively target GBM stem cells (GSCs), and productive infection renders the tumor microenvironment immunostimulatory. We found that: the tyrosine kinase receptor AXL is integral for ZIKV entry into GSCs; the subsequent infection is cytotoxic; and intratumoral injection of ZIKV leads to rapid decrease in tumor volume and prolonged survival in a heterotopic murine patient derived xenograft model. We conclude that ZIKV is a candidate oncolytic virus for the treatment of GBM.
Novocure
Authors
Anat Klein-Goldberg,
Tali Voloshin,
Efrat Zemer-Tov,
Rom Paz,
Lilach Koren,
Kerem Wainer-Katsir,
Alexandra Volodin,
Bella Koltun,
Boris Brant,
Yiftah Barsheshet,
Tal Kan,
Adi Haber,
Brett Mulvey,
Moshe Giladi,
Uri Weinberg,
Yoram Palti
Novocure Ltd, Haifa, Israel
Sensitizing cancer cells to Tumor Treating Fields (TTFields) via PI3K inhibition
Tumor Treating Fields (TTFields) are a non-invasive antimitotic therapy. While TTFields extend life, most patients will eventually progress. This research aimed to identify mechanisms involved with reduced cancer cell sensitivity to TTFields. Glioblastoma and additional cancer cell lines with reduced sensitivity to TTFields were generated in vitro by long-term application of TTFields. Luminex multiplex assay identified that reduced sensitivity was mediated by activation of the PI3K/AKT/mTOR signaling pathway. Application of dual PI3K/mTOR or PI3K inhibitors re-sensitized the cells to TTFields, suggesting concomitant application of TTFields with such inhibitors to improve efficacy.

GlioCure
Authors
Louis-Marie Bachelot*,
Paul Baldwin,
Claire Lépinoux-Chambaud,
Sandrine Courtès*,
Claire Gazaille,
Adelie Mellinger,
Clément Sanchez,
Carl Simonsson
*corresponding author : lm.bachelot@gliocure.com, Sandrine.courtes@gliocure.com
Affiliation: GlioCure
NB: work supported by numerous external collaborations (see logos)
Preclinical development of a neurofilament-derived peptide, GC01 as a safe and specific target of Glioblastoma
GlioCure is a preclinical stage drug development company with a portfolio of 4 products, dedicated to cure glioblastoma. Its lead drug candidate, GC01, is an endogenous peptide that blocks cell division via βIII-tubulin, aberrantly overexpressed in many cancers including brain tumors.
The therapeutic strength of GC01 lies in 1) high tolerability, 2) selectivity of targeting and 3) efficacy against glioblastoma cells and stem cells which are resistant to current treatments and cause recurrence.
GlioCure has secured the development of GC01 towards regulatory preclinical stages and industrial formulation.

Children’s Mercy Kansas City

Authors
Taeju Park,
Neka Large,
Anuradha Roy,
Tom Curran
Children’s Mercy Research Institute, Children’s Mercy Kansas City, Kansas City, Missouri
High Throughput Screening Laboratory, University of Kansas, Kansas
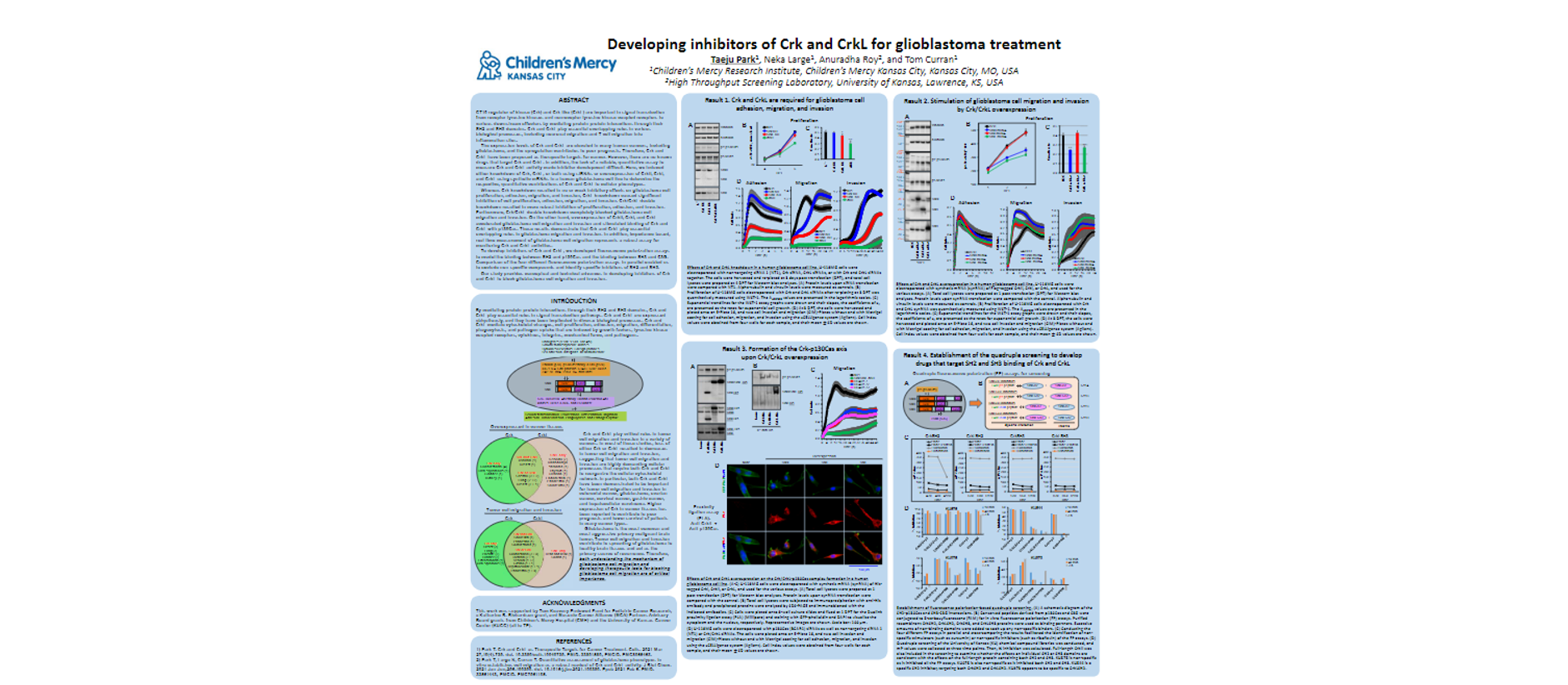
Developing inhibitors of Crk and CrkL for glioblastoma treatment
CT10 regulator of kinase (Crk) and Crk-like (CrkL) are important in signal transduction from receptor tyrosine kinases and nonreceptor tyrosine kinase-coupled receptors to various downstream effectors by mediating protein-protein interactions through their SH2 and SH3 domains. Crk and CrkL play essential overlapping roles in various biological processes, including neuronal migration and T cell migration into inflammation sites.
The expression levels of Crk and CrkL are elevated in many human cancers, including glioblastoma, and the upregulation contributes to poor prognosis. Therefore, Crk and CrkL have been proposed as therapeutic targets for cancer. However, there are no known drugs that target Crk and CrkL. In addition, the lack of a reliable, quantitative assay to measure Crk and CrkL activity made inhibitor development difficult. Here, we induced either knockdown of Crk, CrkL, or both using siRNAs or overexpression of CrkII, CrkI, and CrkL using synthetic mRNAs in a human glioblastoma cell line to determine the respective, quantitative contributions of Crk and CrkL to cellular phenotypes.
Whereas Crk knockdown resulted in no or weak inhibitory effects on glioblastoma cell proliferation, adhesion, migration, and invasion, CrkL knockdown caused significant inhibition of cell proliferation, adhesion, migration, and invasion. Crk/CrkL double knockdown resulted in more robust inhibition of proliferation, adhesion, and invasion. Furthermore, Crk/CrkL double knockdown completely blocked glioblastoma cell migration and invasion. On the other hand, overexpression of CrkII, CrkI, and CrkL accelerated glioblastoma cell migration and invasion and stimulated binding of Crk and CrkL with p130Cas. These results demonstrate that Crk and CrkL play essential overlapping roles in glioblastoma migration and invasion. In addition, impedance-based, real-time measurement of glioblastoma cell migration represents a robust assay for monitoring Crk and CrkL activities.
To develop inhibitors of Crk and CrkL, we developed fluorescence polarization assays to model the binding between SH2 and p130Cas and the binding between SH3 and C3G. Comparison of the four different fluorescence polarization assays in parallel enabled us to exclude non-specific compounds and identify specific inhibitors of SH2 and SH3.
Our study provides conceptual and technical advances in developing inhibitors of Crk and CrkL to block glioblastoma cell migration and invasion.
References:
1) Park T. Crk and CrkL as Therapeutic Targets for Cancer Treatment. Cells. 2021 Mar 27;10(4):739. doi: 10.3390/cells10040739. PMID: 33801580; PMCID: PMC8065463.
2) Park T, Large N, Curran T. Quantitative assessment of glioblastoma phenotypes in vitro establishes cell migration as a robust readout of Crk and CrkL activity. J Biol Chem. 2021 Jan-Jun;296:100390. doi: 10.1016/j.jbc.2021.100390. Epub 2021 Feb 6. PMID: 33561443; PMCID: PMC7961105.
GCAR - Global Coalition for Adaptive Research

Authors
1. Rob Murray – Global Coalition for Adaptive Research
2. Brian Alexander – Foundation Medicine
3. Donald Berry – Berry Consultants, LLC
4. Meredith Buxton – Global Coalition for Adaptive Research
5. Webster Cavenee – University of California, San Diego
6. Howard Colman – Huntsman Cancer Institute
7. John De Groot – University of California, San Francisco
8. Benjamin Ellingson – University of California, Los Angeles
9. Gary Gordon - Global Coalition for Adaptive Research
10. Mustafa Khasraw - Duke Cancer Institute
11. Andrew Lassman - Columbia University Irving Medical Center
12. Eudocia Lee – Dana-Farber Cancer Institute
13. Wenbin Li - Beijing Tiantan Hospital, Capital Medical University
14. Michael Lim – Stanford University School of Medicine
15. Ingo Mellinghoff - Memorial Sloan Kettering Cancer Center
16. Tom Mikkelson - Henry Ford Health System
17. Apoorva Nelli – Global Coalition for Adaptive Research
18. James R. Perry - Sunnybrook Health Sciences Centre
19. Erik Sulman - NYU Langone’s Perlmutter Cancer Center
20. Kirk Tanner – National Brain Tumor Society
21. Michael Weller - University Hospital Zurich
22. Patrick Wen – Dana-Farber Cancer Institute
23. Alfred Yung – MD Anderson Cancer Center
24. Timothy Cloughesy - University of California, Los Angeles
25. GBM AGILE Investigators
GBM AGILE: A Global, Phase 2/3 Adaptive Platform Trial to Evaluate Multiple Regimens in Newly Diagnosed and Recurrent Glioblastoma.
Background: GBM AGILE (Glioblastoma Adaptive, Global, Innovative Learning Environment) is a biomarker based, multi-arm, international, seamless Phase 2/3 Response Adaptive Randomization platform trial designed to rapidly identify experimental therapies that improve overall survival and confirm efficacious experimental therapies and associated biomarker signatures to support new drug approvals and registration.
Operating under a Master Protocol, GBM AGILE allows multiple drugs from different pharmaceutical companies to be evaluated simultaneously and/or over time against a common standard of care control. New experimental therapies are added as new information about promising new drugs is identified while other therapies are removed as they complete their evaluation.
Methods: The evaluation of each experimental therapy in GBM AGILE proceeds in 2 possible stages. A therapy’s Stage 1 is an adaptively randomized screening stage for evaluating the therapy within patient signatures (prospectively defined sets of patient subtypes) compared against a common control arm. If a therapy reaches an efficacy threshold for graduation from Stage 1, it will move into Stage 2 within one of the prospectively defined signatures. For therapies graduating to Stage 2 there is a fixed randomization, expansion cohort.
Results: GBM AGILE received IND approval from the FDA in June 2019, and the first patient was enrolled in July 2019. As of January 2022, GBM AGILE has screened over 1000 patients. Enrollment rates are 3 to 4 times greater than traditional GBM trials, with active sites averaging 0.75 to 1 patients/sites/month. To support global access and approval, GBM AGILE currently has over 45 sites open in the US and Canada with an additional 33 sites across Europe and China forthcoming. Planning is underway for further expansion of GBM AGILE in Australia.
GBM AGILE is a collaboration between academic investigators, patient organizations and industry and serves as a model for more efficient, cost-effective, and accelerated drug development in rare disease.
Clinical trial information: NCT03970447.

IOZK
Authors
Stefaan W. Van Gool,
Jennifer Makalowski,
Michael Bitar,
Peter Van de Vliet,
Volker Schirrmacher,
Wilfried Stuecker,
IOZK, Hohenstaufenring 30-32, 50674 Köln
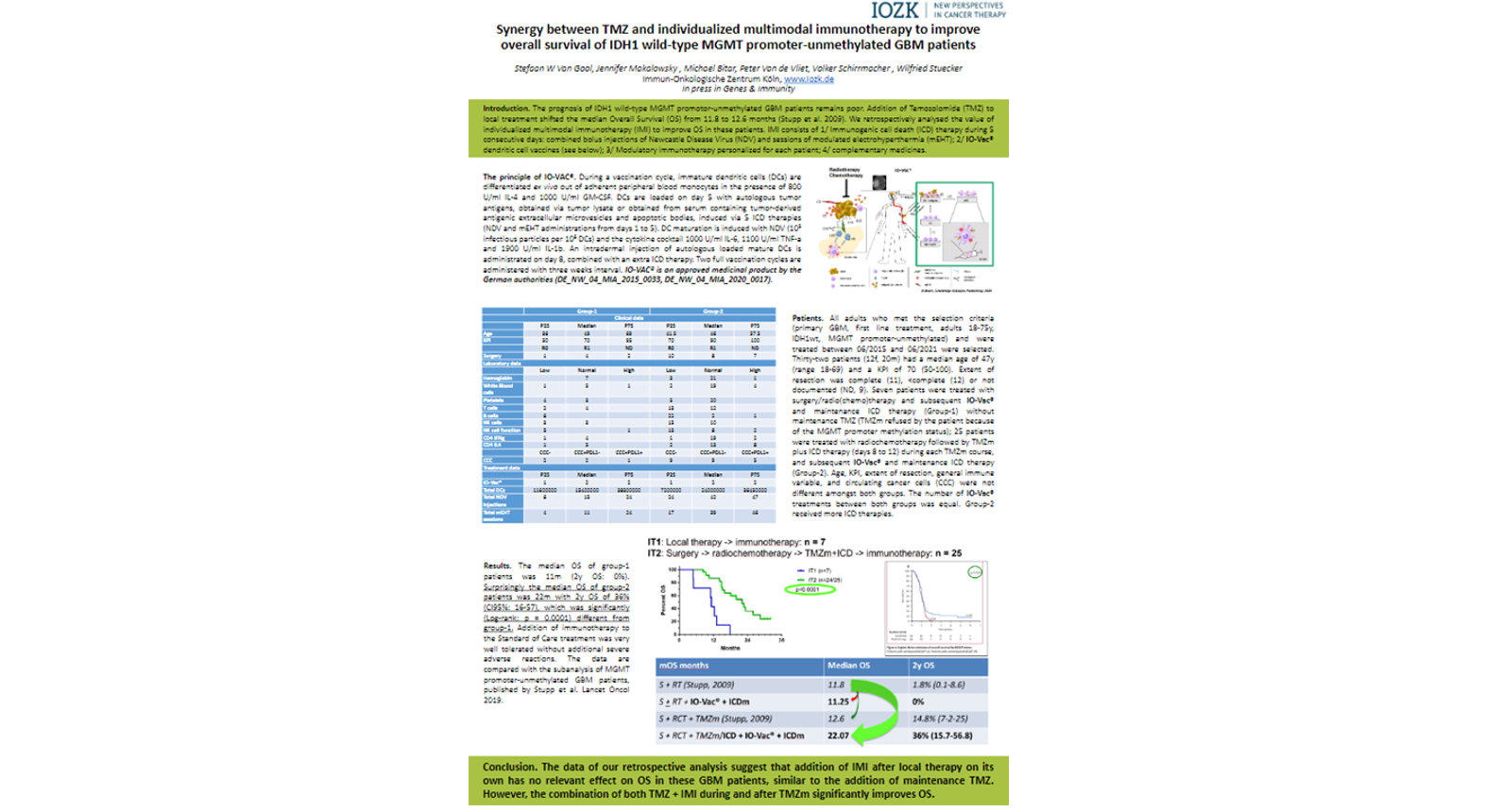
Synergy between TMZ and individualized multimodal immunotherapy to improve Overall Survival of IDH1 wild-type MGMT promoter-unmethylated GBM patients
The prognosis of IDH1 wild-type MGMT promoter-unmethylated GBM patients remains poor. Addition of Temozolomide (TMZ) to first-line local treatment shifted the median overall survival (OS) from 11.8 to 12.6 months. We retrospectively analysed the value of individualized multimodal immunotherapy (IMI) to improve OS in these patients. All adults meeting the criteria and treated 06/2015-06/2021 were selected. Thirty-two patients (12f, 20m) had a median age of 47y (range 18-69) and a KPI of 70 (50-100). Extent of resection was complete (11), <complete (12) or not documented (9). Seven patients were treated with surgery/radio(chemo)therapy and subsequent IMI (Group-1); 25 patients were treated with radiochemotherapy followed by maintenance TMZ plus IMI during and after TMZ (Group-2). Age, KPI and extent of resection were not different amongst both groups. The median OS of group-1 patients was 11m (2y OS: 0%). Surprisingly the median OS of group-2 patients was 22m with 2y OS of 36% (CI95%: 16-57), which was significantly (Log-rank: p = 0.0001) different from group-1. The data suggest that addition of IMI after local therapy on its own has no relevant effect on OS in these GBM patients, similar to maintenance TMZ. However, the combination of both TMZ + IMI significantly improved OS.
Black Diamond Therapeutics

Authors
Melinda Merchant,
Matthew Lucas,
Matthew O’Connor,
Sherri Smith,
Anthony Trombino,
Wu-Yan Zhang,
Jason Simon,
Michael Pickard,
Sudharshan Eathiraj,
Nigel Waters,
Elizabeth Buck
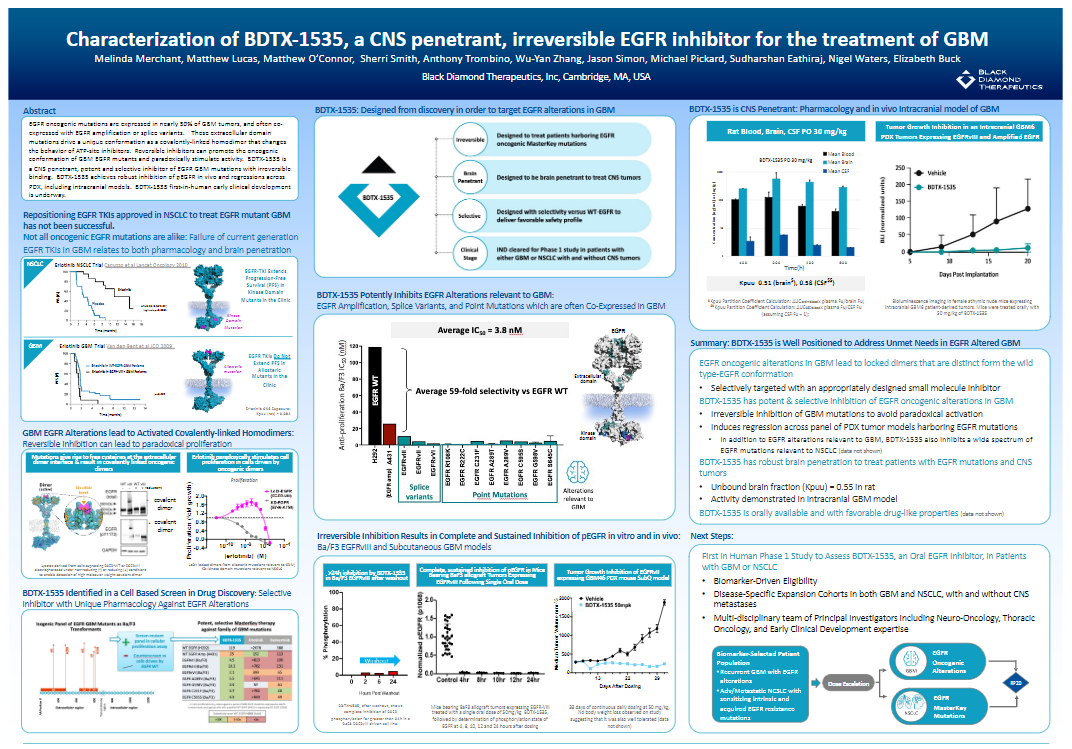
Characterization of BDTX-1535, a CNS Penetrant, Irreversible EGFR Inhibitor for the Treatment of GBM
EGFR oncogenic mutations are expressed in nearly 50% of GBM tumors, and often co-expressed with EGFR amplification or splice variants. These extracellular domain mutations drive a unique conformation as a covalently-linked homodimer that changes the behavior of ATP-site inhibitors. Reversible inhibitors can promote the oncogenic conformation of GBM EGFR mutants and paradoxically stimulate activity. BDTX-1535 is a CNS penetrant, potent and selective inhibitor of EGFR GBM mutations with irreversible binding. BDTX-1535 achieves robust inhibition of pEGFR in vivo and regressions across PDX, including intracranial models. BDTX-1535 first-in-human early clinical development is underway.
National Brain Tumor Society

Driving towards Breakthroughs, Better Health Care and Cures through three interconnected, programmatic strategies: Defeat, Connect, Change.
